CONTRAST Study for Selective Fetal Growth Restriction | Overview
Selective fetal growth restriction (sFGR) occurs when twins sharing a placenta — known as identical, monochorionic twins — experience an uneven distribution of this vital organ. sFGR can result in one fetus not receiving enough nutrients and oxygen and therefore growing slower than the other fetus. Selective fetal growth restriction is reported in about 15 percent of monochorionic twins.
Boston Children's Maternal Fetal Care Center (MFCC), in affiliation with Brigham and Women’s Hospital and in collaboration with Beth Israel Deaconess Medical Center, is taking part in an international, multicenter study examining ways to better predict how the fetuses affected by sFGR will do as they grow in utero. The “selective fetal growth restriction monoChOrioNic Twins – an inteRnAtional inveSTigation” (CONTRAST) study is a joint effort between Boston Children’s Hospital, Leiden University Medical Centre, the UZ Leuven, Karolinska University Hospital), BCNatal Fetal Medicine Research Center, and Mount Sinai Hospital (Toronto, Canada). The study has been approved by the Institutional Review Board (IRB#: IRB-P00046170) and registered under the ClinicalTrials.gov identifier NCT05952583.
What are the objectives of the CONTRAST study?
The primary goal of the CONTRAST study is to improve the prediction of outcomes in pregnancies affected by sFGR by developing a predictive model at the time of diagnosis. The model will aim to enable and strengthen our ability to predict the outcome of pregnancies affected by sFGR.
Study goals:
- Evaluate fetal and neonatal survival
- Evaluate the neonatal morbidity
- Evaluate the long-term neurodevelopment outcomes of sFGR twins
- Evaluate the impact of sFGR on the mental health of parents/caregivers
- Increase our understanding of the pathophysiology (disease process) of sFGR through close examination of the placenta
Why should I participate in the CONTRAST study?
Monochorionic twin pregnancies are at substantial risk of experiencing adverse prenatal conditions, such as sFGR. The MFCC is a Center of Excellence with extensive experience in managing complicated twin pregnancies. In addition, because sFGR is a rare condition, it can be best studied through a multicenter collaboration where the expertise of multiple centers from around the world is gathered together.
Most of the evaluations performed in this study will be integrated as part of our standard (clinical) care for sFGR twins. In addition, your contribution to this study will not only provide you with world-class pregnancy care following the most recent international advancements, but will also significantly contribute to the understating of sFGR that will help affected pregnancies receive the best care possible.
Who is eligible to enroll in the CONTRAST study?
- Pregnant individuals 18 years or older with a monochorionic diamniotic (separate amniotic sacs) twin pregnancy
- Diagnosis of sFGR before 28+0 weeks gestational age
- Partner (if applicable) with present or future parental responsibility who is 18 years or older and capable of giving consent
- Written informed consent of both parents (if applicable) for participation in the longitudinal follow-up until two years after birth
What does participation in the CONTRAST study involve?
The study will consist of three phases:
- Assessment before birth
- Postpartum Assessment
- Long-term follow-up
Here is an overview of the main procedures participants undergo during the study:
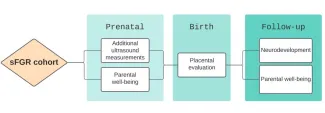
Assessment before birth
Biweekly ultrasound: The ultrasound assessments are classified under either standard or additional ultrasound parameters. The standard assessment is in accordance with the MFCC standard protocol. The additional evaluation is based on the CONTRAST protocol and consists of in-depth evaluations of the babies and placenta. The maximum extra length of the ultrasound examination to obtain the additional measurements is no longer than 10-30 minutes. You will not be charged for the additional assessment.
Parental well-being: We will evaluate maternal and paternal well-being characteristics using standardized questionnaires. These will include questionnaires for the assessment of post-traumatic stress and antenatal attachment. Questionnaires take about 10-20 minutes to complete.
Postpartum assessment
Placental evaluation: The placenta will be examined grossly and microscopically after delivery. This will expand our understanding of placental issues and abnormalities that may be linked to sFGR. The study covers the cost of this evaluation, and you have the option to keep your placenta if you choose.
Neonatal morbidity and delivery outcome: The study team will oversee your babies’ delivery and initial care. We kindly request your consent to allow your physician to share information and data concerning the outcomes of your newborns and your own condition during labor.
Parental well-being: You’ll complete the same questionnaires from your prenatal assessment 6 weeks after delivery.
Long-term follow-up
Neurodevelopmental evaluation: At 24 months, we’ll reach out to you to bring your children for a neurologic and cognitive development assessment using standardized tests (Bayley-IV) at Boston Children’s Hospital Neurodevelopmental Program, conducted by leading experts in the fields of pediatric neurology and neurodevelopment. This assessment takes about one hour, carries no associated risk, and is typically enjoyable for children. The study covers the evaluation cost.
Parental well-being: You’ll complete the same questionnaires from your prenatal assessment at this time.
What if I don’t want to continue being in the CONTRAST?
You can leave the study at any time for any reason.
How do I enroll?
Maternal Fetal Care Center referring physicians and eligible patients can contact 617-355-6512. For questions about the study, please email CONTRASTStudy@childrens.harvard.edu.
We will ask for medical information, including demographic and insurance information, which can be faxed to 617-730-0124 or 617-730-0302, or emailed to CONTRASTStudy@childrens.harvard.edu.
Our team
Principal Investigator
Read more about Alireza Shamshirsaz, MD
Chief, Division of Maternal-Fetal Medicine and Surgery; Director, Maternal Fetal Care Center; Director, Perinatal Surgery Fellowship
Professor of Surgery; Professor of Obstetrics and Gynecology and Reproductive Biology, Harvard Medical School
Coordinating Investigator
Read more about Ali Javinani, MD
Postdoctoral Research Fellow, Maternal Fetal Care Center (MFCC)
Research Fellow in Surgery, General Surgery, Harvard Medical School
Fetal Surgery
Read more about Alireza Shamshirsaz, MD
Chief, Division of Maternal-Fetal Medicine and Surgery; Director, Maternal Fetal Care Center; Director, Perinatal Surgery Fellowship
Professor of Surgery; Professor of Obstetrics and Gynecology and Reproductive Biology, Harvard Medical School
Read more about Eyal Krispin, MD
Attending Physician, Maternal Fetal Care Center
Assistant Professor of Surgery & Assistant Professor of Obstetrics, Gynecology and Reproductive Biology, Harvard Medical School
Maternal-Fetal Medicine
Read more about Cassandra Duffy, MD, MPH
Faculty, Maternal-Fetal Care Center
Instructor of Obstetrics, Gynecology and Reproductive Biology, Harvard Medical School
Read more about Scott Shainker, DO, MS
Faculty, Maternal Fetal Care Center
Assistant Professor, Obstetrics, Gynecology and Reproductive Biology, Harvard Medical School
Radiology
Read more about Patricia Ellen Grant, MD
Director of the Fetal Neonatal Neuroimaging and Developmental Science Center (FNNDSC); Director of Faculty Affairs, Department of Radiology; Director of Research, Maternal Fetal Care Center
Käthe Beutler, MD Harvard Professor of Pediatrics; Professor of Radiology, Harvard Medical School
Read more about Judy A. Estroff, MD
Neonatal Section Chief, Department of Radiology; Staff Radiologist, Department of Radiology
Associate Professor of Radiology, Harvard Medical School
Read more about Ryne Didier, MD
Fetal Section Chief, Department of Radiology
Read more about Esra Abaci Turk, PhD
Associate Scientific Researcher
Instructor of Pediatrics, Harvard Medical School
Fetal Cardiology
Read more about Wayne Tworetzky, MD
Director, Fetal Cardiology Program; Benderson Chair, Boston Children's Hospital; Senior Associate Cardiologist, Department of Cardiology
Associate Professor of Pediatrics, Harvard Medical School
Read more about Danielle Sganga, MD
Cardiologist, Department of Cardiology
Instructor of Pediatrics, Harvard Medical School
Perinatal Pathology
Read more about Katte Carreon, MD
Co-Director, Cardiac Registry; Director of Perinatal & Placental Pathology; General Pediatric Pathology, Department of Pathology
Assistant Professor of Pathology, Harvard Medical School
Neurodevelopment
Read more about Jonathan S. Litt, MD, MPH
Director, NICU GraDS; Physician in Medicine, Division of Newborn Medicine
Assistant Professor of Pediatrics, Harvard Medical School
Read more about Ellen Hanson, PhD
Director, Neurodevelopmental Disorders Developmental Program; Attending Psychologist, Division of Developmental Medicine
Assistant Professor of Pediatrics; Instructor of Psychology, Harvard Medical School
Nursing
Read more about Olivia (Beaudouin) Oppel, RN, BSN, CPN, CLC
Clinical Coordinator, Division of Maternal-Fetal Medicine and Surgery
Bonnie Zapolin, RNC, WHNP-BC
Read more about Nicole Peace, MSN, APRN, FNP-BC
Nurse Practitioner, Maternal Fetal Care Center
Research Specialist
Brittany Gudanowski
Social Work
Read more about Kassie Merrill-Olver, MSW, LICSW
Social Work Program Manager; Clinical Social Worker III, Maternal Fetal Care Center
