Study Details | Overview
Study Details
Why?
Very low blood pressure can be a medical emergency, and if not treated quickly can be life-threatening or cause harm to vital organs like the heart, brain, and kidneys. In order to find the best possible treatment for very low blood pressure, studies need to be done to give doctors information on what dose of epinephrine works best. Many different doses work well, but we need your help to find the best dose.
What we know
From previous studies at Boston Children's Hospital, we know that our ICU clinicians use many different initial doses of epinephrine for very low blood pressure. Most often, doctors use doses between 0.5 mcg/kg to 1 mcg/kg as the initial dose and then adjust later doses based on the response of the blood pressure. Unfortunately, the existing information is not able to tell us what dose is best, but initial doses between 0.5 to 1 mcg/kg seemed to benefit patients similarly.
Who?
Patients hospitalized in the Medical ICU or Medical/Surgical ICU who are 25 years or younger are eligible to participate if their blood pressure becomes dangerously low and the ICU team thinks epinephrine will help.
How?
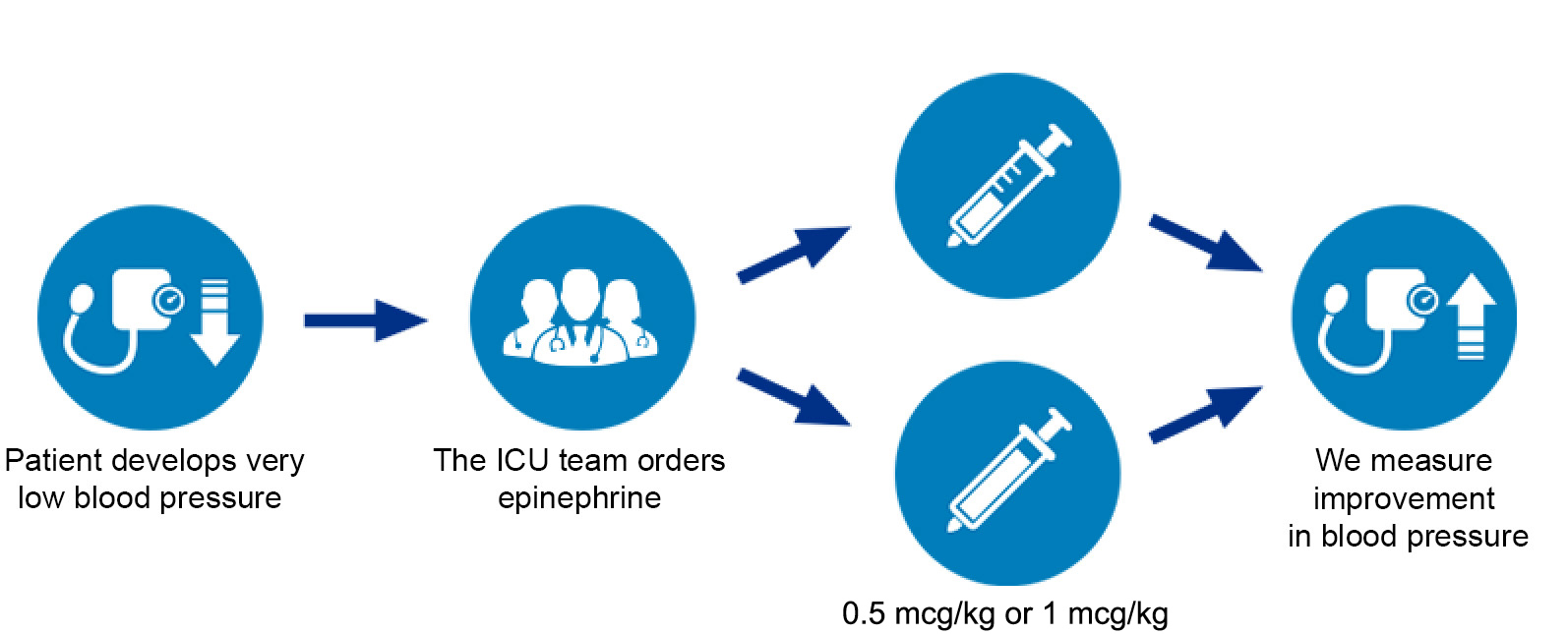
If a patient’s ICU team determines that they need epinephrine for life-threatening low blood pressure, the patient may be enrolled in the EPI Dose Study immediately. Patients who need epinephrine will have a 50:50 chance of receiving EITHER 0.5 mcg/kg OR 1 mcg/kg as the initial dose. The effects on blood pressure and other patient information will be collected from the chart. No other interventions or participation is required.
Safety
It is important to understand that patients enrolled in the EPI Dose Trial would have received epinephrine regardless of being in the study or not; the only difference is the chance of getting one dose or the other as opposed to your doctor choosing which dose to give. Also, the study only affects the first dose of epinephrine needed; if more epinephrine is needed, your doctor will decide how much to give. If your doctor is not satisfied with the increase in blood pressure after the first dose, he/she may give as much additional medication or adjust future doses as necessary.
Your child’s safety will continue to be the top priority.
For more detailed information on the EPI Dose Study, click here.
|
Possible Risk/Side Effect |
How often has it occurred? |
How serious is it? |
Can it be corrected? |
|
Blood pressure does not go up enough. |
Common with either dose of epinephrine. However, we do not know if it is more common with one dose versus the other. |
If prolonged, low blood pressure can damage vital organs including the brain and heart and can sometimes cause death. |
Often the low blood pressure can be corrected with higher doses of epinephrine or other treatments as part of intensive care. However, if there is organ damage, these effects may be irreversible. |
|
Blood pressure goes up too high. |
Occasional with either dose of epinephrine. However, we do not know if it is more common with one dose versus the other. |
For most children and young adults, temporary blood pressure does not cause damage. However, extremely high blood pressure even for a short period could cause organ damage including bleeding in the brain and can sometimes cause death. |
Yes. High blood pressure after epinephrine usually goes away on its own within minutes without any apparent long-term effects. However, if there is organ damage or bleeding in the brain from extremely high blood pressure, these effects may be irreversible. |
Exception From Informed Consent and the EPI Dose Study
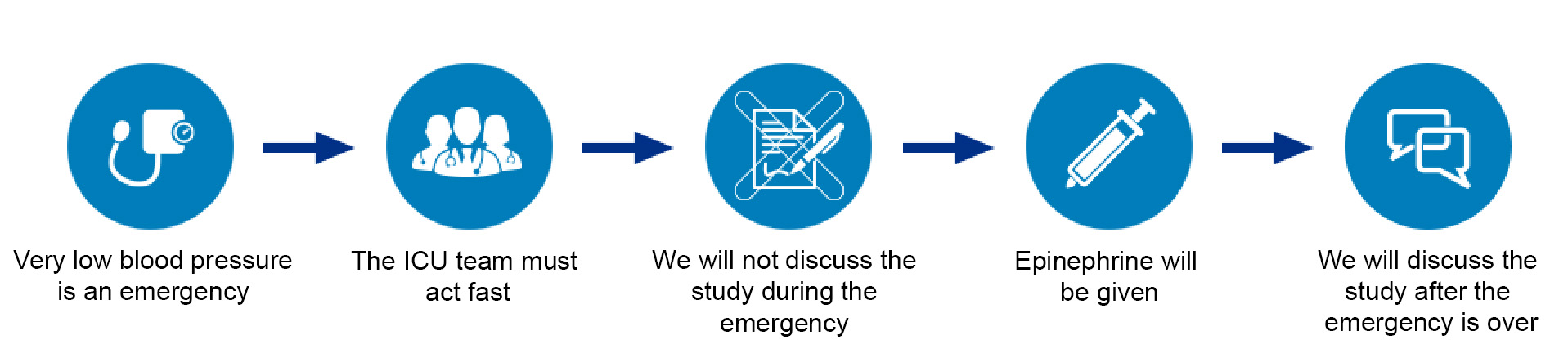
“Exception From Informed Consent” is a special set of government rules for clinical trials that involve life-threatening medical emergencies. During these emergencies, a full discussion about study enrollment and permission to participate, or “informed consent,” is not possible; however, many of these patients could potentially benefit from being in the study. Therefore, these special rules allow for the emergency research to proceed without informed consent but require that extra safeguards are in place to ensure that most people in the community would consider the study to be appropriate.
In short, Exception From Informed Consent can be used when:
- The person’s life is at risk, AND
- The best treatment is not known, AND
- The study might help the person, AND
- It is not possible to get permission for participation because the emergency must be treated as quickly as possible.
In the EPI Dose Study, very low blood pressure is a medical emergency, and the ICU team has to act very quickly to give treatments, including epinephrine. We will do our best to make families aware of the study beforehand and give them the option to “opt out” if they do not want their child to participate. These efforts include giving informational fliers to every family upon admission to the ICU, attempting to visit each family in person or by phone and providing additional information on this website. Additionally, before researchers can start a study using EFIC, they must provide information about the study to the community and get their feedback. For the EPI Dose Study, we have performed surveys and focus groups with our staff and with families whose child has been admitted to the ICU. We welcome any additional feedback which can be provided by contacting us.