Intraocular inflammation and Regeneration | Overview
Intraocular inflammation, oncomodulin, and optic nerve regeneration.
Due to its accessibility, simple anatomy, and functional importance, the optic nerve has been widely studied for insights into positive and negative regulators of axon regeneration in the CNS. We earlier discovered that intraocular inflammation enables retinal ganglion cells (RGCs), the projection neurons of the eye, to undergo dramatic changes in their program of gene expression, revert to an active growth state, and regenerate lengthy axons through the injured optic nerve1-3. We identified Oncomodulin (Ocm), a small Ca2+-binding protein, as a key mediator of these effects, and showed that Ocm binds to a high-affinity receptor on RGCs in a cAMP-dependent manner. Levels of Ocm mRNA and protein increase dramatically in the eye following intraocular inflammation, and gain-of-function and loss-of-function studies show that neutrophil-derived Ocm plays a central role in inflammation-induced regeneration4-6. Our studies also showed that other factors from inflammatory cells are required to enable Ocm to bind to its receptor and to promote RGC survival, and we recently identified a second protein that works synergistically with Ocm. Combining these molecules may enable us to promote regeneration in a clinically relevant manner.
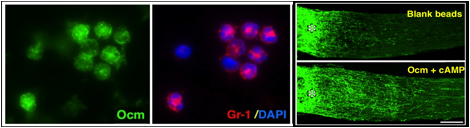
Combinatorial treatments for long-distance axon regeneration and innervation of central target areas.
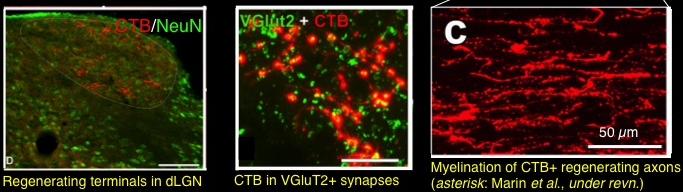
In other studies, we demonstrated a massive synergy between activating RGCs’ growth state via intraocular inflammation and knocking out the gene for pten, a potent cell-intrinsic suppressor of growth10. PTEN acts as a brake on activation of the PI3 kinase-Akt pathway, which controls cell growth, and deletion of PTEN was previously shown to be sufficient to cause appreciable regeneration on its own11. We then showed that combining intraocular inflammation, elevation of cAMP and PTEN deletion enables some RGCs to regenerate axons the full length of the optic nerve, reinervate the appropriate central target areas in the brain, and restore simple visual responses 12. This study represented the first to demonstrate the possibility of restoring the appropriate circuitry of the visual system after optic nerve damage.
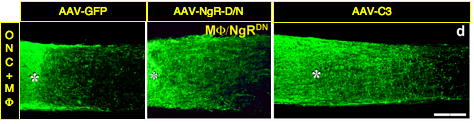
Counteracting cell-extrinsic suppressors of axon growth.
Proteins associated with CNS myelin and the scar that forms at the site of a CNS injury inhibit axon growth in culture, yet counteracting the effects of these proteins has generally been found to be insufficient to promote extensive axon regeneration in vivo. Using a gene-therapy approach, we found that two genetic manipulations that render RGCs unresponsive to myelin and the glial scar failed to induce extensive axon regeneration after optic nerve injury. These treatments included virally-mediated expression of either a dominant-negative form of the Nogo receptor or of C3 ribosyltransferase to inactivate RhoA, a key part of the intracellular signal transduction pathway leading to growth arrest). However, when we combined methods to counteract cell-extrinsic suppressors of axon growth with methods to activate RGCs’ intrinsic growth state, we obtained 3-5 times more regeneration than the latter treatment alone3, 7. Subsequent collaborative studies showed that deleting multiple isoforms of the Nogo receptor, along with PTP-sigma, one of several receptors for inhibitory molecules associated with the scar that forms at an injury site, enable somewhat greater regeneration than seen in our earlier studies, but that again, the effects of counteracting receptors for cell-extrinsic inhibitors of axon growth was greatly augmented by activating RGCs’ intrinsic growth state8, 9. Together, these studies reinforce the insufficiency of counteracting inhibitory proteins as a means of promoting CNS regeneration in vivo while at the same time showing the synergistic effects of combining the latter approach with methods that activate neurons’ intrinsic growth state.
Ongoing projects
- Understanding the cell signaling pathways and sequence of transcriptional changes that underlie successful regeneration in vivo
- altering expression of genes identified thorugh this work to enhance levels of optic nerve regeneration
- identifying the receptor through which Ocm induces axon outgrowth
- developing translationally applicable methods to restore vision after optic nerve injury.
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. J Neurosci Lens injury stimulates axon regeneration in the mature rat optic nerve 2000;20(12):4615-26.
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Journal of Neuroscience Macrophage-derived factors stimulate optic nerve regeneration 2003;23:2284-93.
- Fischer D, Petkova V, Thanos S, Benowitz LI. J Neurosci Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation 2004;24:8726-8740.
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Nat Neurosci Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells 2006;9(6):843-52. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16699509
- Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, Zack DJ, Benowitz LI. Proc Natl Acad Sci U S A Oncomodulin links inflammation to optic nerve regeneration 2009;106(46):19587-92. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19875691.2780793
- Kurimoto T, Yin Y, Habboub G, Gilbert HY, Li Y, Nakao S, Hafezi-Moghadam A, Benowitz LI. J Neurosci Neutrophils express oncomodulin and promote optic nerve regeneration 2013;33(37):14816-24. http://www.ncbi.nlm.nih.gov/pubmed/24027282.3771038
- Fischer D, He Z, Benowitz LI. J Neurosci Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state 2004;24(7):1646-51. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14973241.
- Wang X, Hasan O, Arzeno A, Benowitz LI, Cafferty WB, Strittmatter SM. Exp Neurol Axonal regeneration induced by blockade of glial inhibitors coupled with activation of intrinsic neuronal growth pathways 2012;237(1):55-69. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22728374.3418451
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. Nat Neurosci NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans 2012;15(5):703-12. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22406547.3337880
- Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, Moko L, Kugler S, Benowitz LI. J Neurosci Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion 2010;30(46):15654-63. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21084621.3001271
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Science Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway 2008;322(5903):963-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18988856.2652400
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L. Proc Natl Acad Sci U S A Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors 2012;109(23):9149-54. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22615390.
