Retinopathy of Prematurity | Overview
Retinopathy of Prematurity in Humans
- Retinopathy of prematurity (ROP) is a major cause of blindness in children in the developing and developed world despite current surgical treatment in the late stage of the disease
- In the US ROP affects approximately 14 million infants and results in approximately 1,100-1,500 babies requiring treatment each year.
Causes of Retinopathy of Prematurity
- ROP is associated with excessive oxygen use shortly after birth. When human babies are born prematurely, they are often given supplemental oxygen to ensure adequate blood levels of oxygen. However, this can induce ROP. Human preterm infants with ROP show delayed development of the retinal vasculature, which can be associated with pathological neovascularization.
- The harmful neovascularization from ROP can damage the retina and lead to vision loss and blindness.
- Currently the two major risk factors for ROP appear to be low gestational weight of the baby and oxygen use, although elevated glucose may also play a role
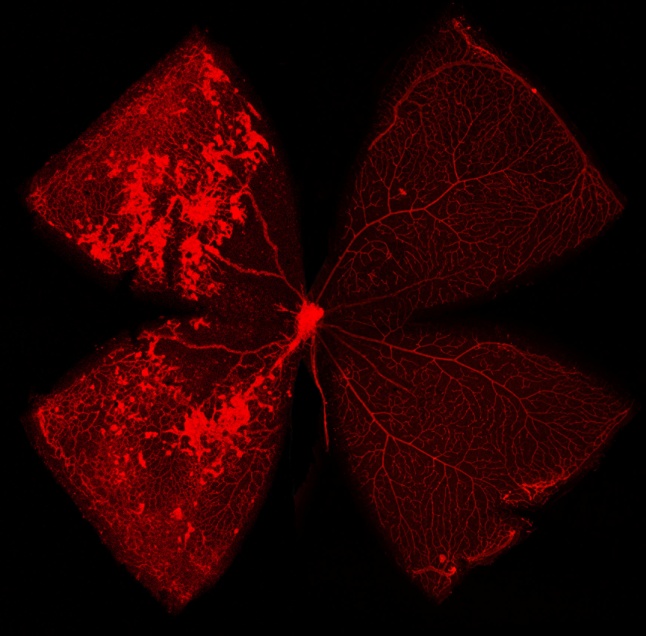
Above: A merged image of a hyperoxic mouse retina (left) with a normoxic retina (right) at P17. Note the neovascular tufts and vaso-obliteration on the left.
- Silverman W (1980) Retrolental fibroplasia: a modern parable. Grune and Stratton, New York
- http://www.aapos.org/terms/conditions/94
Oxygen-Induced Retinopathy in The Mouse
- Neonatal mice and their nursing mother are kept at room air from birth through postnatal day (P)7.
- From P7 to P12, the mice are exposed to 75% oxygen, which induces loss of immature retinal vessels and slows development of the normal retinal vasculature, leading to a central zone of vaso-obliteration (VO).
- The mice are returned to room air. At this point, the central avascular retina becomes hypoxic, triggering both normal vessel regrowth and a pathologic formation of extraretinal neovascularization (NV).
- Maximum severity of NV is reached at P17. Shortly thereafter, NV starts to regress and by P25 almost no VO or NV remains visible.
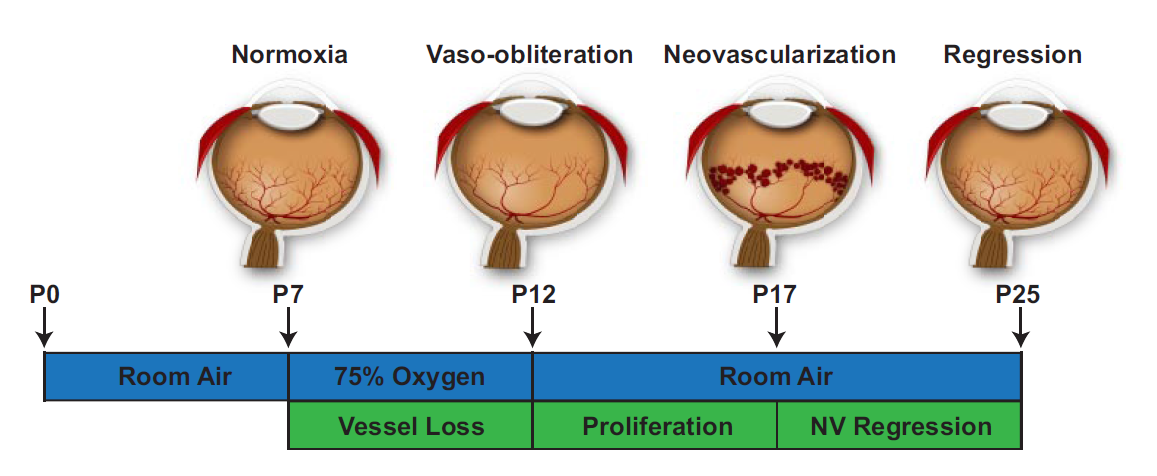
Above: A Representative Diagram of our model of OIR
We have found that VEGF, EPO and IGF-1 all contribute to the development of this harmful neovascularization (see Selected Publications). Our lab is currently investigating the role of the WNT, the JAK-STAT, and the FEVR pathways in the OIR model. Members of our lab have recently published papers investigating the role of SOCS3 and Cyp2C8 in our OIR model and we hope to use our knowledge of these pathways to develop treatments and therapies for ROP in humans.
