Breadcrumb
- Home
- Conditions & Treatments
- Tricuspid Atresia
What is tricuspid atresia?
Tricuspid atresia is a congenital heart defect, occurring in two out of every 10,000 live births. Relatively rare, it accounts for about 1 to 2 percent of all cases of congenital heart disease.
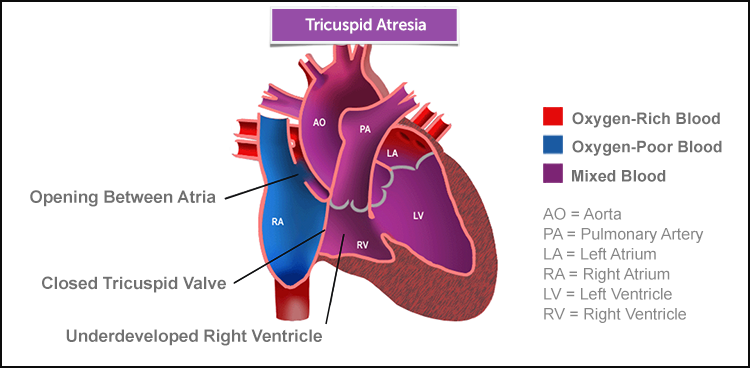
In tricuspid atresia, the tricuspid valve — which is normally located between the right atrium and the right ventricle — is missing, as is the right ventricle. While very serious, tricuspid atresia is treatable surgically, and surgical advances have greatly improved outcomes.
In a normal heart, oxygen-poor (blue) blood returns to the right atrium from the body, travels to the right ventricle, then is pumped through the pulmonary artery into the lungs, where it receives oxygen. And oxygen-rich (red) blood returns to the left atrium from the lungs, passes into the left ventricle, then is pumped through the aorta out to the body.
In tricuspid atresia, lack of development of the tricuspid valve prevents oxygen-poor (blue) blood from passing from the right atrium to the right ventricle and on to the lungs as it should. Instead, blood passes from the right atrium to the left atrium and then to the only pumping chamber, the left ventricle. In most children with tricuspid atresia, the artery to the lungs is connected to the left ventricle by a ventricular septal defect.
Additional defects
Like many congenital heart conditions, tricuspid atresia isn’t a single defect, but rather a cluster of associated defects in various combinations and with varying degrees of severity.
Essentially all children with atrial septal defect and most have ventricular septal defect (VSD). These defects are holes in the tissue walls that normally separate the right and left atria (ASD) and the right and left ventricles (VSD).
These ASDs and VSDs are actually useful in this condition, since they allow oxygen-poor (blue) blood and oxygen-rich (red) blood to mix, providing some oxygen to circulate. The ASD allows blood returning from the body to enter the single pumping chamber (the left ventricle), where the blood is pumped to the lungs and to the body. The VSD allows blood to be pumped to the the lungs from the left ventricle. But the heart often has to work extra hard with this set-up, which is not as efficient as having two pumping chambers.
Some children may also have a defect called patent ductus arteriosus. In this condition, a blood vessel (ductus arteriosus), which connects the two great arteries (aorta and pulmonary artery), and which usually closes soon after birth, fails to do so. The ductus arteriosus remains open (patent), allowing blood to pass from the aorta to the pulmonary artery. This is helpful if the VSD is very small or there is narrowing of the pulmonary valve, since it allows enough blood to get to the lungs for enough oxygen. But the ductus is subject to closing without warning, and children who need a patent ductus arteriosus for adequate blood flow to the lungs, require it to be replaced by a shunt.
Some children also have transposition of the great arteries (TGA), in which the normal positions of the aorta and pulmonary artery are reversed. These children often also have narrowing of the major blood vessel going to the body (the aorta).
Symptoms & Causes
What are the symptoms of tricuspid atresia?
The symptoms of tricuspid atresia may include:
- Rapid breathing
- Rapid heartbeat
- Blue color of the skin, lips, and nailbeds (cyanosis)
- Sweating
- Disinterest in feeding or tiring while feeding
- Poor weight gain
- Heart murmur (detected by doctor)
These symptoms also can be caused by other conditions, and some can be found in perfectly normal babies and children.
What causes tricuspid atresia?
Most often, tricuspid atresia occurs sporadically with no clear reason for its development. Some congenital heart defects may have a genetic link, causing heart problems to occur more often in certain families.
Diagnosis & Treatments
How is tricuspid atresia diagnosed?
Tricuspid atresia is usually diagnosed before birth with a fetal ultrasound or in the first few days of life. If your newborn baby was born with a bluish tint to the skin or other concerning symptoms, your pediatrician will refer you to a pediatric cardiologist, who will perform a physical exam along with some combination (not necessarily all) of the following medical tests:
How is tricuspid atresia treated?
Specific treatments for tricuspid atresia depend on the associated cardiac conditions and other variables. Most newborn babies with the condition are admitted to the intensive care unit (ICU) or special care nursery. Initially, your child may be placed on oxygen or (less commonly) a ventilator to help with breathing and may be given IV (intravenous) medications to help the heart and lungs function more efficiently.
Once your child is stabilized, your baby's treatments will probably include:
Cardiac catheterization
Prior to the initial surgery, or between staged operations (see below), doctors occasionally need to perform a cardiac catheterization procedure, although this is seldom needed in newborns with tricuspid atresia.
Medication
Doctors may administer an IV (intravenous) medication to prevent the closing of the infant's ductus arteriosus — the prenatal connection between the aorta and the pulmonary artery, which usually closes shortly after birth, but is in some babies important as a temporary alternative opening for blood flow to the lungs.
Surgery
Since tricuspid atresia with an underdeveloped right ventricle is considered a single ventricle defect (where the defect results in just one fully functioning ventricle), it's usually treated using single ventricle palliation — a staged series of three operations performed between the first few days or months and the first few years of life. The first stage is to optimize the blood flow to the lungs, whether it's too much or too little. If the blood flow is too little, a Blalock-Taussig-Thomas (BTT) shunt is usually performed. If it's too much, the pulmonary artery may be banded to control blood flow. If it's neither too little nor too much, the first stage may be skipped, and the second stage — the bi-directional Glenn — may be performed at 4 to 8 months of age.
For babies with tricuspid atresia and transposition of the great arteries and narrowing of the the aorta, a more complex operation, where the pulmonary artery is connected to the aorta, the aorta enlarged and a BTT shunt is placed, may be required.
Blalock-Taussig-Thomas (BTT) shunt
When the blood flow to the lungs is inadequate, this operation is usually performed a few days after birth to create a pathway for blood to reach the lungs. A small tube (about 3½ mm in diameter) made of Gore-tex material is connected to a branch of the aorta and the pulmonary artery. Some of the blood traveling through the aorta towards the body will “shunt” through this connection and flow into the pulmonary artery to receive oxygen.
Pulmonary artery banding (PAB)
This alternative first operation, done when the blood flow to the lungs is excessive, is performed to reduce and limit pulmonary artery blood flow. This is to prevent excessive work by the left ventricle, and to protect the small blood vessels in the lung from damage.
Bi-directional Glenn
The second operation, often performed when a child is between about 4 and 6 months old, reduces the left ventricle's workload and sets the stage for the Fontan procedure to come. This procedure replaces the BTT shunt (which the baby's heart will outgrow) with another connection to the pulmonary artery. The superior vena cava (the large vein that returns oxygen-poor blood from the head and arms back to the heart) is surgically connected to the right pulmonary artery so that blood can proceed directly to the lungs to receive oxygen.
Fontan procedure
This final operation in the sequence, done in the first few years of life, is performed for treatment of children with various types of single ventricle defects, including tricuspid atresia. The principle of the procedure is that it's not necessary to have a ventricle that pumps to the lungs, so long as small blood vessels in the lung are healthy and blood can flow through the lung at low pressure. It is necessary for the ventricle to readily accept blood coming back from the lungs and to function well. The surgery involves directly connecting the returning blue blood from both the upper and lower parts of the body into the pulmonary arteries. This can be achieved in a number of different ways — sometimes with, and sometimes without, the use of synthetic tubes (conduits).
Biventricular repair
In some instances, your baby’s single ventricle heart can be converted into two functioning ventricles (biventricular circulation). This repair can be the initial procedure or single ventricle palliation may be used before converting the heart to biventricular circulation.
Home Monitoring Program
An infant with a single ventricle needs somewhat closer monitoring and support than one with two ventricles. Research shows the vital importance of a Home Monitoring Program, including daily at-home assessments of oxygen saturations and weight between the stage I (neonatal) and stage II (bi-directional Glenn) surgeries. We have a very experienced Home Monitoring Program to provide these special babies and their families the support that they need.
What is the long-term outlook for tricuspid atresia?
Surgical techniques for tricuspid atresia and its associated defects are continually being refined with long-term outcomes continually improving. Nevertheless, children with tricuspid atresia will need lifelong monitoring and medication, since they will always be at some risk for arrhythmias, infections, heart failure, or stroke.
Your cardiologist will help you create a long-term care program as your baby grows into childhood, the teen years and even adulthood. Most people who've had congenital heart disease repair will have an ongoing relationship with their cardiologist. We'll help prevent and treat complications and will advise on daily-life issues, such as activity levels, nutrition, and precautions related to pregnancy.
How we care for tricuspid atresia
The Boston Children’s Hospital Department of Cardiac Surgery treats some of the most complex pediatric heart conditions in the world, such as tricuspid atresia, with survival rates exceeding 98 percent.
We provide families with a wealth of information, resources, programs and support — before, during and after your child’s treatment. With our compassionate, family-centered approach to expert treatment and care, your family and your child are in the best possible hands.
The Boston Adult Congenital Heart (BACH) and Pulmonary Hypertension program provides long-term inpatient and outpatient care and advanced therapeutic options for patients with congenital heart disease and pulmonary hypertension as they move through adulthood.




