Breadcrumb
- Home
- Programs & Services
- Fetal Care and Surgery Center
- Research & Innovation
- CONTRAST Study For Selective Fetal Growth Restriction
CONTRAST Study for Selective Fetal Growth Restriction
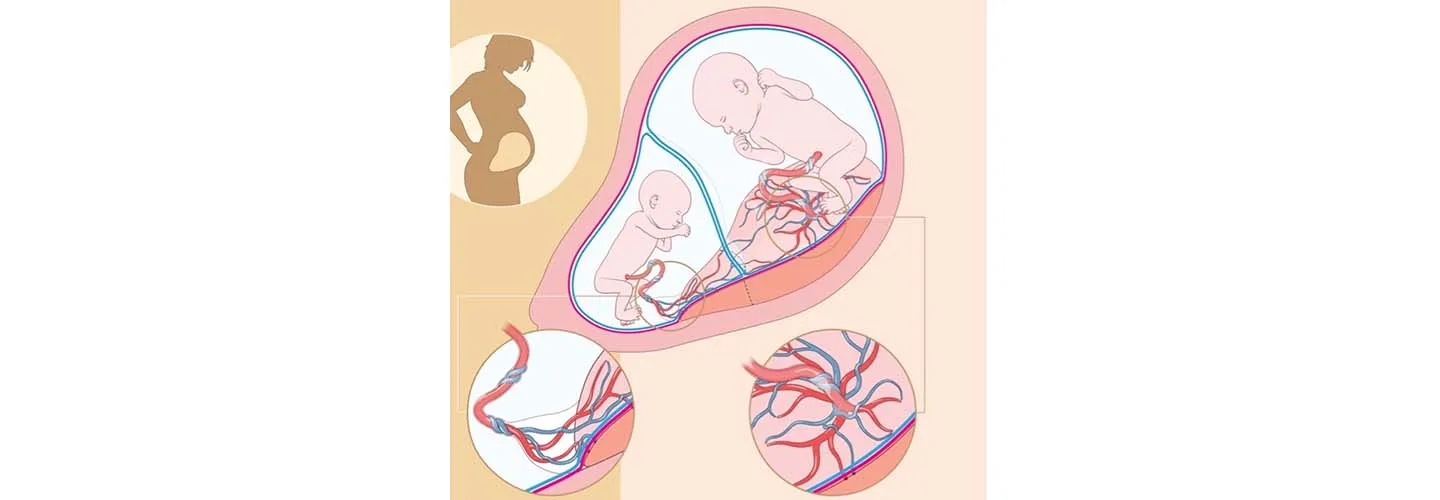
Selective fetal growth restriction (sFGR) occurs when twins sharing a placenta — known as identical, monochorionic twins — experience an uneven distribution of this vital organ. sFGR can result in one fetus not receiving enough nutrients and oxygen and therefore growing slower than the other fetus. Selective fetal growth restriction is reported in about 15 percent of monochorionic twins.
Boston Children's Fetal Care and Surgery Center (FCSC), in affiliation with Brigham and Women’s Hospital and in collaboration with Beth Israel Deaconess Medical Center, is taking part in an international, multicenter study examining ways to better predict how the fetuses affected by sFGR will do as they grow in utero. The “selective fetal growth restriction monoChOrioNic Twins — an inteRnAtional inveSTigation” (CONTRAST) study is a joint effort between Boston Children’s Hospital, Leiden University Medical Centre, the UZ Leuven, Karolinska University Hospital), BCNatal Fetal Medicine Research Center, and Mount Sinai Hospital (Toronto, Canada). The study has been approved by the Institutional Review Board (IRB#: IRB-P00046170) and registered under the ClinicalTrials.gov identifier NCT05952583.
How do I enroll in the study?
Fetal Care and Surgery Center referring physicians and eligible patients can contact 617-355-6512. For questions about the study, please email CONTRASTStudy@childrens.harvard.edu.
We will ask for medical information, including demographic and insurance information, which can be faxed to 617-730-0124 or 617-730-0302, or emailed to CONTRASTStudy@childrens.harvard.edu.
What are the objectives of the CONTRAST study?
The primary goal of the CONTRAST study is to improve the prediction of outcomes in pregnancies affected by sFGR by developing a predictive model at the time of diagnosis. The model will aim to enable and strengthen our ability to predict the outcome of pregnancies affected by sFGR.
Study goals:
- Evaluate fetal and neonatal survival
- Evaluate the neonatal morbidity
- Evaluate the long-term neurodevelopment outcomes of sFGR twins
- Evaluate the impact of sFGR on the mental health of parents/caregivers
- Increase our understanding of the pathophysiology (disease process) of sFGR through close examination of the placenta
Why should I participate in the CONTRAST study?
Monochorionic twin pregnancies are at substantial risk of experiencing adverse prenatal conditions, such as sFGR. The FCSC is a Center of Excellence with extensive experience in managing complicated twin pregnancies. In addition, because sFGR is a rare condition, it can be best studied through a multicenter collaboration where the expertise of multiple centers from around the world is gathered together.
Most of the evaluations performed in this study will be integrated as part of our standard (clinical) care for sFGR twins. In addition, your contribution to this study will not only provide you with world-class pregnancy care following the most recent international advancements, but will also significantly contribute to the understating of sFGR that will help affected pregnancies receive the best care possible.
Who is eligible to enroll in the CONTRAST study?
- Pregnant individuals 18 years or older with a monochorionic diamniotic (separate amniotic sacs) twin pregnancy
- Diagnosis of sFGR before 28+0 weeks gestational age
- Partner (if applicable) with present or future parental responsibility who is 18 years or older and capable of giving consent
- Written informed consent of both parents (if applicable) for participation in the longitudinal follow-up until two years after birth
What does participation in the CONTRAST study involve?
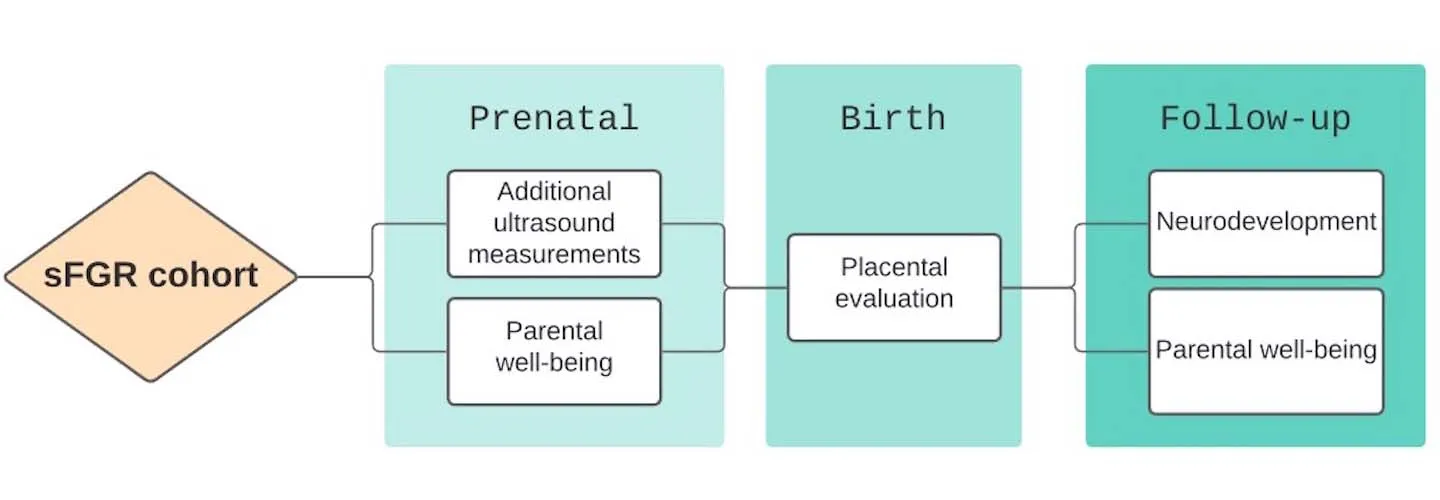
The study will consist of three phases:
- Assessment before birth
- Postpartum assessment
- Long-term follow-up
Here is an overview of the main procedures participants undergo during the study:
Assessment before birth
- Biweekly ultrasound: The ultrasound assessments are classified under either standard or additional ultrasound parameters. The standard assessment is in accordance with the FCSC standard protocol. The additional evaluation is based on the CONTRAST protocol and consists of in-depth evaluations of the babies and placenta. The maximum extra length of the ultrasound examination to obtain the additional measurements is no longer than 10 to 30 minutes. You will not be charged for the additional assessment.
- Parental well-being: We will evaluate maternal and paternal well-being characteristics using standardized questionnaires. These will include questionnaires for the assessment of post-traumatic stress and antenatal attachment. Questionnaires take about 10 to 20 minutes to complete.
Postpartum assessment
- Placental evaluation: The placenta will be examined grossly and microscopically after delivery. This will expand our understanding of placental issues and abnormalities that may be linked to sFGR. The study covers the cost of this evaluation, and you have the option to keep your placenta if you choose.
- Neonatal morbidity and delivery outcome: The study team will oversee your babies’ delivery and initial care. We kindly request your consent to allow your physician to share information and data concerning the outcomes of your newborns and your own condition during labor.
- Parental well-being: You’ll complete the same questionnaires from your prenatal assessment 6 weeks after delivery.
Long-term follow-up
- Neurodevelopmental evaluation: At 24 months, we’ll reach out to you to bring your children for a neurologic and cognitive development assessment using standardized tests (Bayley-IV) at Boston Children’s Hospital Neurodevelopmental Program, conducted by leading experts in the fields of pediatric neurology and neurodevelopment. This assessment takes about one hour, carries no associated risk, and is typically enjoyable for children. The study covers the evaluation cost.
- Parental well-being: You’ll complete the same questionnaires from your prenatal assessment at this time.
What if I don’t want to continue being in the CONTRAST?
You can leave the study at any time for any reason.
Our team
Principal investigator
Alireza Shamshirsaz , MD
Coordinating investigator
Ali Javinani , MD
Ehsan Rojhani , MD
Fetal surgery
Alireza Shamshirsaz , MD
Eyal Krispin , MD
Maternal-fetal medicine
Cassandra Duffy , MD, MPH
Scott Shainker , DO, MS
Radiology
Patricia Ellen Grant , MD
Judy A. Estroff , MD
Ryne Didier , MD
Esra Abaci Turk , PhD
Fetal cardiology
Wayne Tworetzky , MD
Danielle Sganga , MD
Perinatal pathology
Katte Carreon , MD
Neurodevelopment
Ellen Hanson , PhD
Nursing
Olivia (Beaudouin) Oppel , RN, BSN, CPN, CLC
Bonnie Zapolin , MS, RNC, WHNP-BC
Nicole Peace , MSN, APRN, FNP-BC
Research specialist




















