Now that we know RNAs can thrive on the cell surface, a new frontier of possibilities is unfolding. What can RNA do from its perch on the top of the cell that it cannot do from inside it and how can its position there be used to our advantage?
Dr. Flynn points out that RNA is a flexible platform. It can bind to molecules, shut down or enhance cell-to-cell communication, or help cells hide from the immune system. Because it is so versatile, Dr. Flynn thinks we should be able to tweak it in ways that can help us combat disease.
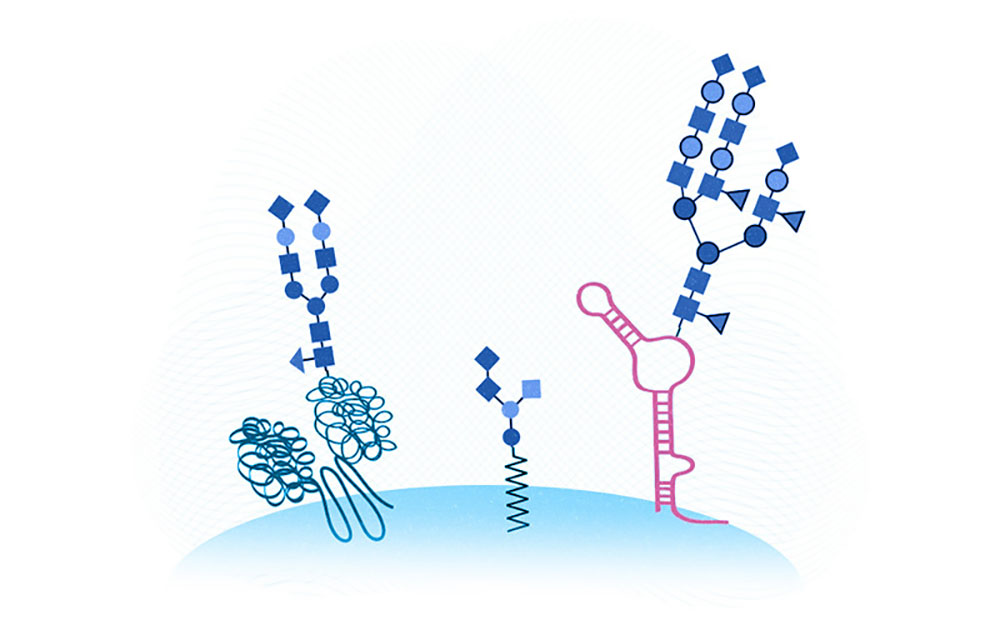
We now have three ‘hands’ on the cell surface. From left to right, a glycoprotein, a glycolipid, and the newly discovered glycoRNA. (Illustration: Ryan Flynn/Sebastian Stankiewicz, Boston Children’s Hospital)
"For example, blood cancers may have too much sugar RNA [glycoRNA] on the cell surface and that could cause cancer. We could develop a therapy to cut the RNA off the cell surface, reducing cancer growth," he says.
In short, by developing glycoRNA-based therapies, we may be able to address a myriad of diseases by enhancing or blocking cell-surface glycoRNAs on diseased cells.
RNA and autoimmune disease: A topic for the future
Our cell surfaces are covered in lipids, sugars and proteins that help transmit signals back and forth. But sometimes unwanted visitors invade, triggering immune cells to swoop in and attack. In autoimmune diseases, such as lupus, RNAs can be mislabeled as "imposters” and assaulted.