Project 1. The mechanisms of immune-vascular interaction in controlling retinopathy
Inflammation and changes in immune function are clearly involved in retinal angiogenesis, but the way to control inflammation is not clear. Immune cells are a source of cytokines and growth factors that may interact with the endothelial cells and contribute to the abnormalities of the vessels. There is increasing evidence for the critical role of immune cells in retinal vascular development, remodeling, repair, and anastomosis. Suppressor of cytokine signaling 3 (SOCS3) is a critical regulator that controls innate and adaptive immunity, tissue inflammation, cytokine production, and macrophage polarization. Our lab is actively pursuing the mechanisms of essential immune-regulators, including SOCS3, that mediate immune-vascular interaction in ocular neovascularization formation using mouse models of retinopathy of prematurity and age-related macular degeneration. (See Figure 1)
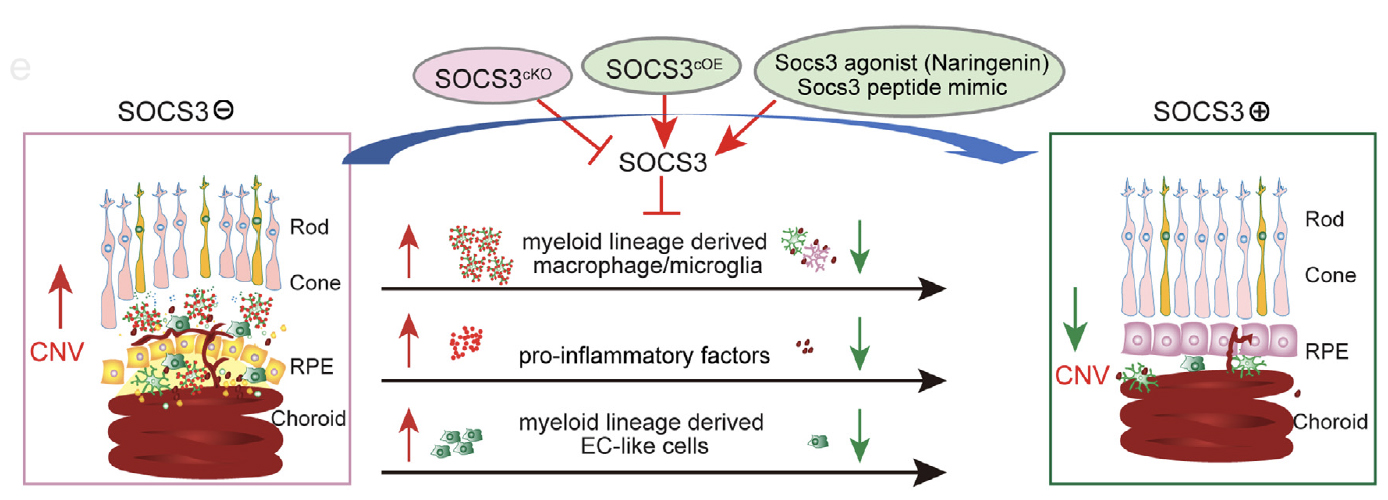
Figure 1.
Project 2. Photoreceptors determination of retinal blood vessel growth in retinopathy
In retinopathy of prematurity, the onset of neovascularization coincides with the development of rod outer segments and therefore full rod function, suggesting an important role of photoreceptors in ROP pathogenesis. Yet the molecular and cellular mechanisms through which photoreceptors control retinal vascularization in retinopathy of prematurity are largely unknown. c-Fos is an immediate early gene and pro-oncogene that acts as a master regulator of many inflammatory factors. c-Fos encodes a nuclear phosphoprotein that forms heterodimeric complexes with Jun-family molecules to form activator protein 1 complex, which regulates many inflammatory signals in diseases. Our lab focuses on the roles of c-Fos pathway as a major signaling pathway that is used by stressed photoreceptors to convey a need for blood vessels and as a potential upstream target to control the development of retinopathy. (See Figure 2)
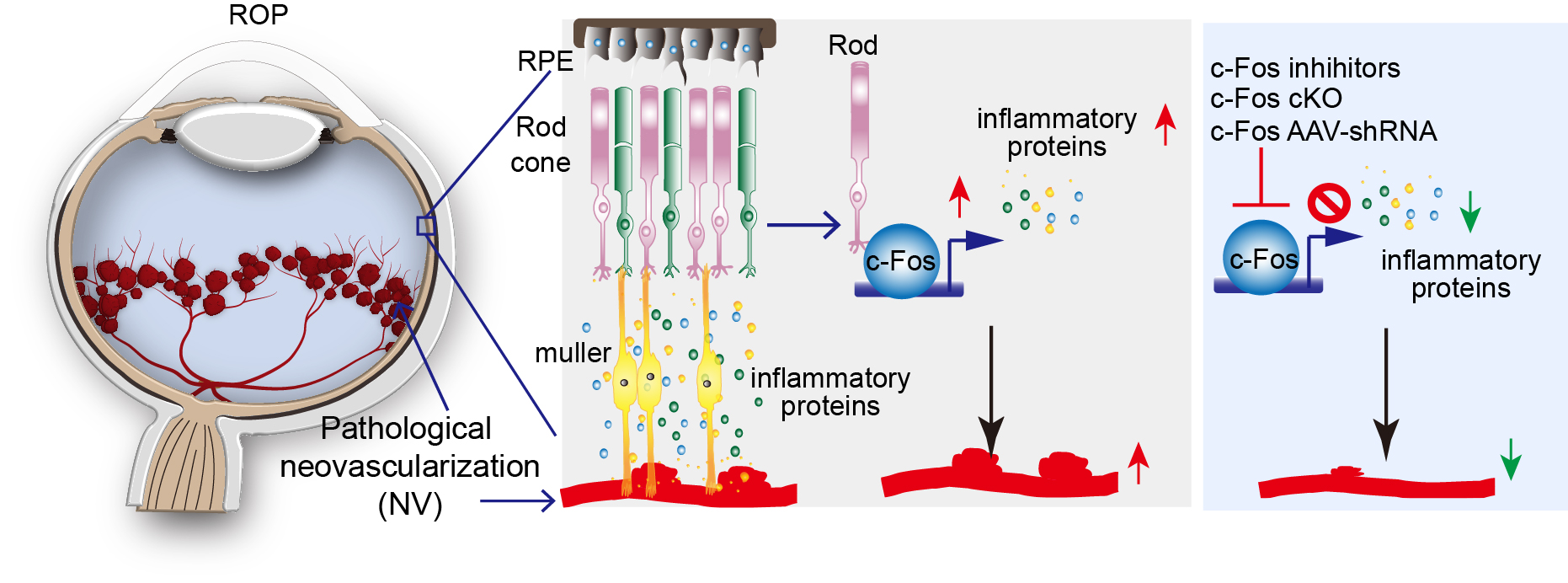
Figure 2.
Project 3. Immune regulation in retinal degeneration
Common to all retinal degenerative diseases is the damage to photoreceptors that malfunction and degenerate, as in age-related macular degeneration and retinitis pigmentosa. Currently there is no treatment for inherited photoreceptor degeneration. Retinal degenerative diseases are heterogeneous diseases, it is unlikely to develop an effective approach by replacing or correcting only one single defective gene. The long-term goal is to identify new treatments that target a pathway common to various retinal degenerative diseases. The photoreceptor layer is immune privileged, in which adaptive immunity and inflammation are tightly controlled, and loss of immune privilege leads to diseases. Our lab currently focuses on the studies to determine the roles of the immune regulators (such as SOCS3 and c-Fos) during the development of photoreceptor degeneration.