Based upon the observations by many surgeons that removal and replacement (ACL reconstruction) does not preserve the long-term function of the joints, we determined it essential to understand the mechanisms that underlie the inability of an injured ACL to heal so that we can design novel clinically relevant ways to repair it.
By understanding how to affect an efficient repair of a torn ligament, the need to remove and replace it would be obviated and progressive joint failure (post-traumatic OA) might be prevented. Therefore, we designed a series of experiments aimed at understanding why a torn ACL does not heal.
These experiments would differentiate between intrinsic cellular deficiency of ACL fibroblasts, vascular deficiency, and local environmental factors, such as the bathing of the damaged ends of the ligament by synovial fluid or an inability to bring apposing ends of the torn ligament sufficiently close together using suturing or other stabilizing techniques.
We began by comparing ACL fibroblasts(1) with those obtained from the medial collateral ligament (MCL),(2;3) which is an extra-articular ligament that has no difficulty healing with conservative “non-surgical” treatment with a brace. By combining in vitro cell culture assays with in vivo histologic and immunohistochemical techniques, we found that MCL and ACL cells within injured ligaments have comparable rates of proliferation and that each ligament is able to revascularize after rupture.(2;1;3) We also observed comparable collagen production within the ligaments up to one year after injury.(4) Importantly, however, we made the germinal observation that the provisional scaffold found in the wound site of the MCL and other extra-articular ligaments, was missing in the ACL (Fig 1).
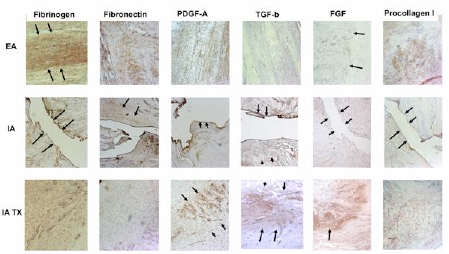
Figure 1: Photomicrographs of extra-articular (EA; first and third rows), untreated intra-articular (IA; second and fourth rows) 21 and 42 days after wounding. All photomicrographs taken at 10X. Immunohistochemistry (where brown or red denotes a positive presence of the molecule of interest) revealed active and functional wound sites in the extra-articular wounds at both time point; however, the untreated IA ligament wounds remain relatively empty of any substratum. Adapted from Murray et al, JOR 2007.
Among the potential explanations for poor healing response of the ACL was the difference in mechanical stabilization between the ACL, and the altered environments surrounding these torn ligaments (Fig 2). The ACL is surrounded by synovial fluid, whereas the MCL and all other extraarticular ligaments are not. Thus, while the cells and vascularity of the ACL were capable of mounting the same functional healing response, there was no structure in place to rejoin the two ends of the ligament for the cells to invade and remodel (Fig 1). These findings(5; 6;7;8) led us to hypothesize that the lack of provisional scaffold between the two ends of the torn ACL was the key mechanism behind the failure of this joint tissue to heal (Fig 2).
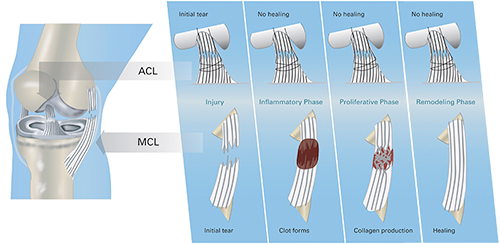 Figure 2: Differences in the intrinsic healing response of the ACL (top row) and medial collateral ligament of the knee (MCL, bottom row). The ACL is injured, but no blood clot forms in the injury site, likely due to the synovial fluid which bathes the ligament washing the clot out. In contrast, in the MCL, blood clot forms at the site of the tear and stabilizes the two ligament ends. The MCL tissue can then grow into this provisional scaffold and the defect can be healed. The loss of the provisional scaffold in the ACL is likely the key mechanism behind its failure to heal.
Figure 2: Differences in the intrinsic healing response of the ACL (top row) and medial collateral ligament of the knee (MCL, bottom row). The ACL is injured, but no blood clot forms in the injury site, likely due to the synovial fluid which bathes the ligament washing the clot out. In contrast, in the MCL, blood clot forms at the site of the tear and stabilizes the two ligament ends. The MCL tissue can then grow into this provisional scaffold and the defect can be healed. The loss of the provisional scaffold in the ACL is likely the key mechanism behind its failure to heal.
The observation that there was essentially no provisional scaffold formation or wound site filling between the ends of the ruptured human ACL led us to hypothesize that there would be a quantitative difference in the amount of wound site filling in the ACL and MCL even in a mechanically stable and contained defect(8). We additionally hypothesized that any molecular deposition within the wound site would differ between the ACL and the MCL – specifically that the presence of fibrinogen, fibronectin, PDGF-A, TGF-β1, FGF-2 and vWF would be different in the two wound sites(9;10).
To test this hypothesis, a canine model was used. Central defects were created in extra-articular ligaments (MCL and/or patellar ligament) and an intra-articular ligament (ACL) in canine knees and the histologic response to injury evaluated at 3 days (n=3), 7 days (n=4), 21 days (n=5) and 42 days (n=5).
The findings in these studies were that MCL and PL defects exhibited far greater filling of the wound site and increased presence in the wound site of fibrinogen, fibronectin, PDGF-A, TGF-β1, FGF-2 and vWF when compared to ACL defects at all time points(10). Thus, this study supported the hypothesis that there is a lack of provisional scaffold found in the intra-articular wound site of the ACL, and this loss is also associated with a decreased presence of important extracellular matrix proteins and cytokines within the wound site of the ACL.
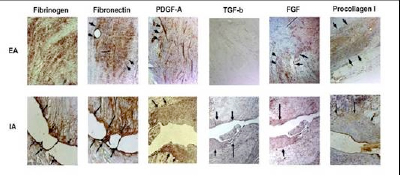 Figure 3: Representative micrographs of the wound site in the extra-articular patellar ligament (EA row) and the intra-articular ACL (IA row) after three weeks in vivo. Note the filling of the wound site in the EA ligament with an active repair process occurring within the provisional scaffold (top row). In contrast, the ACL wounds remain unfilled (bottom row)(10).
Figure 3: Representative micrographs of the wound site in the extra-articular patellar ligament (EA row) and the intra-articular ACL (IA row) after three weeks in vivo. Note the filling of the wound site in the EA ligament with an active repair process occurring within the provisional scaffold (top row). In contrast, the ACL wounds remain unfilled (bottom row)(10).
References
- Murray, MM, Martin, SD, Martin, TL, Spector, M. 2000. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am 82: 1387-1397.
- Murray, MM, Spindler, KP, Ballard, P, et al. 2007. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res 25: 1007-1017.
- Frank, C, Schachar, N, Dittrich, D. 1983. Natural history of healing in the repaired medial collateral ligament. J Orthop Res 1: 179-188.
- Spindler, KP, Clark, SW, Nanney, LB, Davidson, JM. 1996. Expression of collagen and matrix metalloproteinases in ruptured human anterior cruciate ligament: an in situ hybridization study. J Orthop Res 14: 857-861.
- Murray, MM, Martin, SD, Martin, TL, Spector, M. 2000. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am 82-A: 1387-1397.
- Murray, MM, Spector, M. 2001. The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials 22: 2393-2402.
- Murray, MM, Spindler, KP, Ballard, P, et al. 2007. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res 25: 1007-1017.
- Spindler, KP, Murray, MM, Devin, C, et al. 2006. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res 24: 401-406.
- Murray, MM, Spindler, KP, Devin, C, et al. 2006. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res 24: 820-830.
- Murray, MM, Spindler, KP, Ballard, P, et al. 2007. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res.