Intraoperative localization of cardiac conduction tissue regions using real-time fibre-optic confocal microscopy: first in human trial.
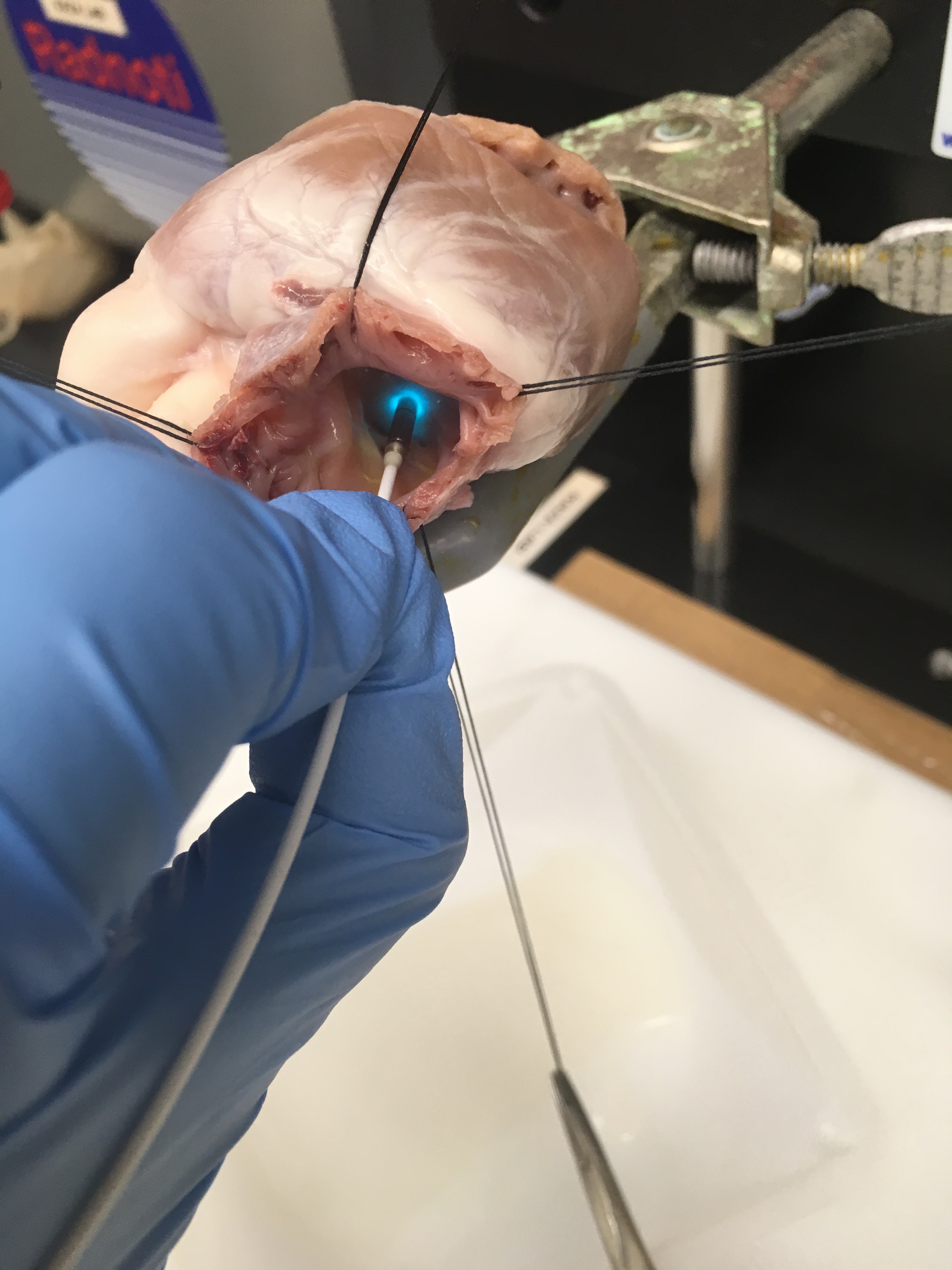 The aim of this study was to evaluate the feasibility and safety of fibre-optic confocal microscopy (FCM) using fluorescein sodium dye for the intraoperative location of conduction tissue regions during paediatric heart surgery. The pilot study included 6 patients undergoing elective surgery for the closure of isolated secundum atrial septal defect aged 30 days to 21 years. FCM imaging was integrated within the normal intraoperative protocol for atrial septal defect repair. Fluorescein sodium dye was applied on the arrested heart. FCM images were acquired at the atrioventricular node region, sinus node region and right ventricle (RV). Total imaging time was limited to 3 min. Any adverse events related to the study were recorded and analysed. Subjects received standard postoperative care. Trained reviewers (n = 9) classified, de-identified and randomized FCM images (n = 60) recorded from the patients as presenting striated, reticulated or indistinguishable microstructures. The reliability of reviewer agreement was assessed using Fleiss' kappa. Reticulated myocardial microstructures were found during FCM imaging at atrioventricular node and sinus node regions, while striated microstructures were observed in RV. Reliability of agreement of reviewers classifying the FCM images was high (Fleiss' kappa: 0.822). FCM using fluorescein sodium dye was found to be safe for use during paediatric heart surgery. The study demonstrates the potential for FCM to be effective in identifying conduction tissue regions during congenital heart surgery.
The aim of this study was to evaluate the feasibility and safety of fibre-optic confocal microscopy (FCM) using fluorescein sodium dye for the intraoperative location of conduction tissue regions during paediatric heart surgery. The pilot study included 6 patients undergoing elective surgery for the closure of isolated secundum atrial septal defect aged 30 days to 21 years. FCM imaging was integrated within the normal intraoperative protocol for atrial septal defect repair. Fluorescein sodium dye was applied on the arrested heart. FCM images were acquired at the atrioventricular node region, sinus node region and right ventricle (RV). Total imaging time was limited to 3 min. Any adverse events related to the study were recorded and analysed. Subjects received standard postoperative care. Trained reviewers (n = 9) classified, de-identified and randomized FCM images (n = 60) recorded from the patients as presenting striated, reticulated or indistinguishable microstructures. The reliability of reviewer agreement was assessed using Fleiss' kappa. Reticulated myocardial microstructures were found during FCM imaging at atrioventricular node and sinus node regions, while striated microstructures were observed in RV. Reliability of agreement of reviewers classifying the FCM images was high (Fleiss' kappa: 0.822). FCM using fluorescein sodium dye was found to be safe for use during paediatric heart surgery. The study demonstrates the potential for FCM to be effective in identifying conduction tissue regions during congenital heart surgery.
Kaza, A, Mondal, A, Piekarski, B, Sachse, F, Hitchcock, R, Intraoperative localization of cardiac conduction tissue regions using real-time fibre-optic confocal microscopy: first in human trial, European Journal of Cardio-Thoracic Surgery (2020), https://doi.org/10.1093/ejcts/ezaa040
Preclinical Evaluation of a Pediatric Airway Stent for Tracheobronchomalacia
The primary aim of this study was to demonstrate in an animal model that helical NiTi stents designed for malacic airways could be delivered and removed without significant trauma while minimally impeding mucus clearance during the period of implantation. Stents were delivered and removed from the tracheas of healthy 20 kg swine using tools designed to minimize trauma. In 4-week experiments, the stents were implanted on day 0 and removed after 3 weeks. Weekly bronchoscopies, X-rays and mucus clearance examinations were performed in vivo. Hematoxylin and eosin (H&E) staining and scanning electron microscopy (SEM) imaging were used to evaluate foreign body response, tracheal tissue reaction and damage and to measure non-ciliated regions. In all in vivo experiments, the stent was implanted and removed atraumatically. Mucus clearance was maintained throughout the experiment period. H&E stained slides showed that foreign body response and tracheal tissue damage were localized to the stented subsections. Tracheal tissue reaction and damage was further restricted to the epithelium and submucosal layers. SEM imaging revealed that the cilia was absent only over the contact area between the trachea and the wire forming the helical stent. Helical NiTi stents designed to provide radial support for malacic airways were well tolerated in a porcine model, providing for mucus clearance while also enabling atraumatic removal.
Mondal A, Ha J, Jo VY, Wu F-Y, Kaza AK, Dupont PE, Preclinical Evaluation of a Pediatric Airway Stent for Tracheobronchomalacia, The Journal of Thoracic and Cardiovascular Surgery (2020), doi: https://doi.org/10.1016/j.jtcvs.2020.03.007.
An Imaging Protocol to Discriminate Specialized Conduction Tissue During Congenital Heart Surgery
We performed preclinical validation of intraoperative fiber-optic confocal microscopy (FCM) and assessed its safety and efficacy in an ovine model of the pediatric heart. Intraoperative imaging was performed using an FCM system (Cellvizio, Mauna Kea Technology, Paris, France) with specialized imaging miniprobe (GastroFlex UHD, Mauna Kea Technologies). Before imaging, we applied an extracellular fluorophore, sodium fluorescein, to fluorescently label extracellular space. We imaged arrested hearts of ovine (1-6 months) under cardiopulmonary bypass. Image sequences (1-10 seconds duration) were acquired from regions of the sinoatrial and atrioventricular node, as well as subepicardial and subendocardial working myocardium from atria and ventricle. The surgical process was evaluated for integration of the imaging protocol during the operative procedure. In addition, fluorescein cardiotoxicity studies (n = 3 animals) were conducted by comparing electrocardiogram (PR and QRS intervals) and ejection fraction at baseline and after topical application of fluorescein at 1:10, 1:100, and 1:1000 dilutions on a beating ovine heart. Our studies suggest that intraoperative FCM can be used to identify regions associated with specialized conducting tissue in ovine hearts in situ. The imaging protocol was integrated with conventional open heart surgical procedures with minimal changes to the operative process. Application of fluorescein in varying concentrations did not affect the normalized PR interval, QRS interval, and ejection fraction. These preclinical validation studies demonstrated both safety and efficacy of the proposed intraoperative imaging approach. The studies constitute an important step toward first-in-human clinical trials.
Mondal A, Lackey J, Saeed M, Wu FY, Johnson JK, Huang C, Sachse FB, Hitchcock R, Kaza AK. An Imaging Protocol to Discriminate Specialized Conduction Tissue During Congenital Heart Surgery. Semin Thorac Cardiovasc Surg. 2019 Autumn;31(3):537-546. doi: 10.1053/j.semtcvs.2019.02.006. Epub 2019 Feb 6. PMID: 30738149; PMCID: PMC6684868.
Toward detection of conduction tissue during cardiac surgery: Light at the end of the tunnel?
Postoperative conduction block requiring lifetime pacemaker placement continues to be a considerable source of morbidity for patients undergoing repair of congenital heart defects. Damage to the cardiac conduction system (CCS) during surgical procedures is thought to be a major cause of conduction block. Intraoperative identification and avoidance of the CCS is thus a key strategy to improve surgical outcomes. A number of approaches have been developed to avoid conduction tissue damage and mitigate morbidity. Here we review the historical and contemporary approaches for identification of conduction tissue during cardiac surgery. The established approach for intraoperative identification is based on anatomic landmarks established in extensive histologic studies of normal and diseased heart. We focus on landmarks to identify the sinus and atrioventricular nodes during cardiac surgery. We also review technologies explored for intraoperative tissue identification, including electrical impedance measurements and electrocardiography. We describe new optical approaches, in particular, and optical spectroscopy and fiberoptic confocal microscopy (FCM) for identification of CCS regions and working myocardium during surgery. As a template for translation of future technology developments, we describe research and regulatory pathways to translate FCM for cardiac surgery. We suggest that along with more robust approaches to surgeon training, including awareness of fundamental anatomic studies, optical approaches such as FCM show promise in aiding surgeons with repairs of heart defects. In particular, for complex defects, these approaches can complement landmark-based identification of conduction tissue and thus help to avoid injury to the CCS due to surgical procedures.
Sachse FB, Johnson J, Cottle B, Mondal A, Hitchcock R, Kaza AK. Toward detection of conduction tissue during cardiac surgery: Light at the end of the tunnel? Heart Rhythm. 2020 Jul 10:S1547-5271(20)30643-3. doi: 10.1016/j.hrthm.2020.07.008. Epub ahead of print. PMID: 32659372.
Localization of the sinoatrial and atrioventricular nodal region in neonatal and juvenile ovine hearts
Localization of the components of the cardiac conduction system (CCS) is essential for many therapeutic procedures in cardiac surgery and interventional cardiology. While histological studies provided fundamental insights into CCS localization, this information is incomplete and difficult to translate to aid in intraprocedural localization. To advance our understanding of CCS localization, we set out to establish a framework for quantifying nodal region morphology. Using this framework, we quantitatively analyzed the sinoatrial node (SAN) and atrioventricular node (AVN) in ovine with postmenstrual age ranging from 4.4 to 58.3 months. In particular, we studied the SAN and AVN in relation to the epicardial and endocardial surfaces, respectively. Using anatomical landmarks, we excised the nodes and adjacent tissues, sectioned those at a thickness of 4 μm at 100 μm intervals, and applied Masson's trichrome stain to the sections. These sections were then imaged, segmented to identify nodal tissue, and analyzed to quantify nodal depth and superficial tissue composition. The minimal SAN depth ranged between 20 and 926 μm. AVN minimal depth ranged between 59 and 1192 μm in the AVN extension region, 49 and 980 μm for the compact node, and 148 and 888 μm for the transition to His Bundle region. Using a logarithmic regression model, we found that minimal depth increased logarithmically with age for the AVN (R2 = 0.818, P = 0.002). Also, the myocardial overlay of the AVN was heterogeneous within different regions and decreased with increasing age. Age associated alterations of SAN minimal depth were insignificant. Our study presents examples of characteristic tissue patterns superficial to the AVN and within the SAN. We suggest that the presented framework provides quantitative information for CCS localization. Our studies indicate that procedural methods and localization approaches in regions near the AVN should account for the age of patients in cardiac surgery and interventional cardiology.
Johnson JK, Cottle BK, Mondal A, Hitchcock R, Kaza AK, Sachse FB. Localization of the sinoatrial and atrioventricular nodal region in neonatal and juvenile ovine hearts. PLoS One. 2020 May 7;15(5):e0232618. doi: 10.1371/journal.pone.0232618. PMID: 32379798; PMCID: PMC7205220.