Project One
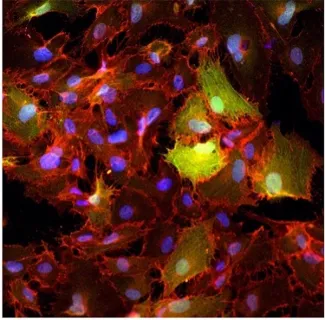
Infantile hemangioma is a vascular tumor that can grow rapidly, causing organ damage, disfigurement and morbidity. The lab has focused on the cellular mechanisms that drive this uncontrolled vascular growth. We isolated increasingly less differentiated cells from hemangioma tissue, which led us to the identification of a multi-potent stem cell that can recapitulate hemangioma in immune-deficient mice. The hemangioma stem cells differentiate into endothelial cells, pericytes and adipocytes, thereby forming the major cell types in this common childhood tumor.
We have three major goals in this project. The first is to understand the mechanisms that control the differentiation of the hemangioma stem cells into endothelial cells, pericytes and adipocytes. The second is to determine if there are somatic mutations or other genetic alterations that contribute to infantile hemangioma. The third is to understand mechanisms of drug action on hemangioma stem cell differentiation and hemangioma blood vessel formation as a basis for potentially new therapies that will act safely and quickly to prevent hemangioma growth.
Project Two
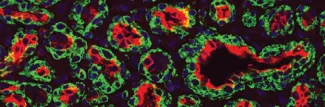
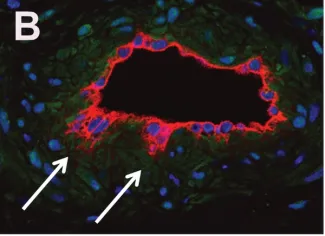
Vascular malformations are defects in the architecture and function of arteries, veins, capillaries and lymphatic vessels. The malformations can be familial or sporadic, and are often associated with tissue overgrowth, deformity, and infections. We focus on capillary malformations (CM), which consist of excessive and abnormal capillary/venule-like vessels. CMs are often on the face and are referred to in lay terms as port-wine birthmarks. Sturge-Weber syndrome (SWS) is a rare neurological disorder in which excessive abnormal vessels akin to CM are found on the surface of the brain and are thought to contribute to the neurologic deficits in SWS.
A somatic, activating mutation in GNAQ (p.R183Q) was discovered in patients with SWS and CM, linking these two disorders. We fractionated CM lesions and SWS brain lesions into specific cell populations that were then genotyped for the GNAQ (p.R183Q) mutation. Our studies show that the GNAQ (p.R183Q) mutation is enriched in the endothelial cells from skin and brain CMs. This identification the cellular context in which the GNAQ mutation resides provides essential information needed to decipher the mechanisms by which CMs form, how to prevent CM and how to regress CM.
We have four major goals in this project. The first is to understand how the GNAQ mutation in endothelial cells leads to the excessive and abnormal blood vessels. The second is to determine if GNAQ mutant endothelial cells exert paracrine effects on surrounding cells that are deleterious, the third is to create models that reflect the impacts of mutant GNAQ so that we can screen for drugs to treat SWS, and the fourth is to understand the developmental history of the original cell in which the somatic mutation occurred.
Project Three
We also study endothelial to mesenchymal transition (EndMT). Our earlier work showed that endothelial cells isolated from cardiac valves can recapitulate processes that occur during valve development such as EndMT. We serendipitously discovered that protein tyrosine phosphatase CD45 is expressed in valve endothelial cells undergoing EndMT and that induced CD45 expression can drive EndMT in vascular endothelial cells. We are currently collaborating with Dr. Hong Chen’s lab to determine if and how CD45 might drive EndMT in mouse models of atherosclerosis.