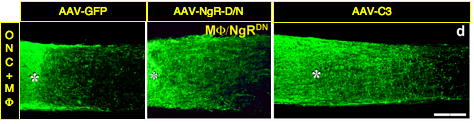
Proteins associated with CNS myelin and the scar that forms at the site of a CNS injury inhibit axon growth in culture, yet counteracting the effects of these proteins has generally been found to be insufficient to promote extensive axon regeneration in vivo. Using a gene-therapy approach, we found that two genetic manipulations that render RGCs unresponsive to myelin and the glial scar failed to induce extensive axon regeneration after optic nerve injury. These treatments included virally mediated expression of either a dominant-negative form of the Nogo receptor or of C3 ribosyltransferase to inactivate RhoA, a key part of the intracellular signal transduction pathway leading to growth arrest. However, when we combined methods to counteract cell-extrinsic suppressors of axon growth with methods to activate RGCs’ intrinsic growth state, we obtained three to five times more regeneration than the latter treatment alone1-2. Subsequent collaborative studies showed that deleting multiple isoforms of the Nogo receptor, along with PTP-sigma, one of several receptors for inhibitory molecules associated with the scar that forms at an injury site, enable somewhat greater regeneration than seen in our earlier studies, but that again, the effects of counteracting receptors for cell-extrinsic inhibitors of axon growth was greatly augmented by activating RGCs’ intrinsic growth state3-4. Together, these studies reinforce the insufficiency of counteracting inhibitory proteins as a means of promoting CNS regeneration in vivo while at the same time showing the synergistic effects of combining the latter approach with methods that activate neurons’ intrinsic growth state.
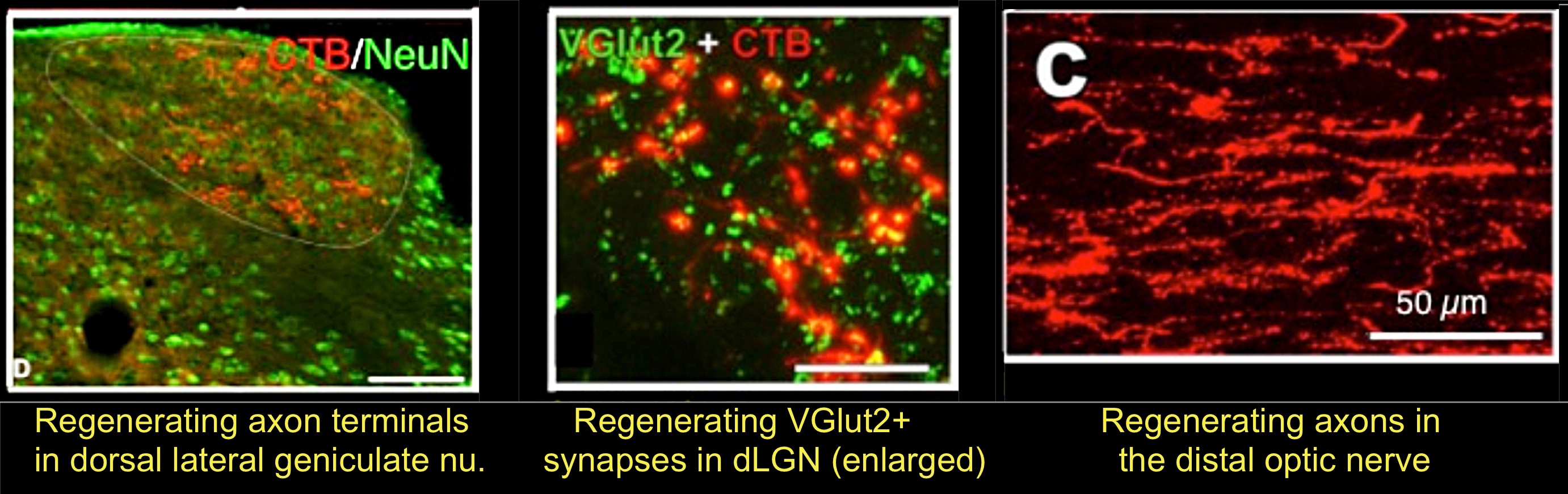
As noted above, there is a remarkable synergy between activating RGCs’ growth state via proteins associated with intraocular inflammation and knocking out the gene for Pten5. PTEN acts as a brake on activation of the PI3 kinase-Akt pathway, which controls cell growth, and deletion of PTEN is sufficient to induce appreciable regeneration6. After eight to 10 weeks of treatment, the combination of intraocular inflammation, cAMP elevation, and PTEN deletion enabled some RGCs to regenerate axons the full length of the optic nerve, reinnervate the appropriate central target areas in the brain, and restore simple visual responses7. This study represents the first to demonstrate the possibility of restoring the appropriate circuitry of the visual system after optic nerve damage, and it was followed by similar findings from other groups8-10.
References
- Fischer, D., He, Z. & Benowitz, L. I. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci 24, 1646-1651, doi:10.1523/JNEUROSCI.5119-03.2004 (2004).
- Fischer, D., Petkova, V., Thanos, S. & Benowitz, L. I. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci 24, 8726-8740 (2004).
- Wang, X. et al. Axonal regeneration induced by blockade of glial inhibitors coupled with activation of intrinsic neuronal growth pathways. Exp Neurol 237, 55-69, doi:10.1016/j.expneurol.2012.06.009 (2012).
- Dickendesher, T. L. et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 15, 703-712, doi:10.1038/nn.3070 (2012).
- Kurimoto, T. et al. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci 30, 15654-15663, doi:10.1523/JNEUROSCI.4340-10.2010 (2010).
- Park, K. K. et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963-966, doi:322/5903/963 [pii] 10.1126/science.1161566 (2008).
- de Lima, S. et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A 109, 9149-9154, doi:10.1073/pnas.1119449109 (2012).
- Lim, J. H. et al. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci 19, 1073-1084, doi:10.1038/nn.4340 (2016).
- Yungher, B. J., Luo, X., Salgueiro, Y., Blackmore, M. G. & Park, K. K. Viral vector-based improvement of optic nerve regeneration: characterization of individual axons' growth patterns and synaptogenesis in a visual target. Gene Ther, doi:10.1038/gt.2015.51 (2015).
- Bei, F. et al. Restoration of Visual Function by Enhancing Conduction in Regenerated Axons. Cell 164, 219-232, doi:10.1016/j.cell.2015.11.036 (2016).
