The research team
Every study has a research team who work together to follow what's called a "protocol" — a detailed plan for the study. The protocol balances the potential benefits and risks to participants, and answer specific research questions.
Research team members include:
- Principal investigator (PI): The PI, who is often a doctor or scientist, is responsible for the entire study. This person plans the study, makes sure activities are done safely and correctly, and decides what each research team member does. The PI supervises all research activities and all of the members of the study team. The PI also makes sure everyone on the team has the proper training.
- Co-Investigators: Many studies have co-investigators — researchers (often doctors or scientists) — who work with the PI. Co-investigators might help the PI design the study or supervise it, but they are not responsible for the entire study.
- Research nurse: A research nurse often has special training and experience. Many studies that involve medical or clinical research have a research nurse on the team who helps carry out the study, focusing on the care and safety of research participants, and is often involved in coordinating daily study activities.
- Research coordinator/research assistant: The research coordinator/research assistant is a person who works with the other research team members to organize and help with daily study tasks.
Other individuals also may help with a research study, such as pharmacists, lab technicians, dieticians who help with participants dietary needs, social workers and office staff.
What are clinical trials?
Clinical trials are medical research studies in which people participate as research volunteers. There are some clinical trials that help researchers understand how a disease or disorder progresses through a person’s life.
Clinical trials are a means of developing treatments, medications, or new approaches for dealing with diseases and conditions.
In a clinical trial, researchers observe the effects an intervention may have on participants. The researcher collects data (information) about how the intervention affects participants’ health. The intervention may be a medical drug or device or procedure; it might also be a behavior change that participants agree to try.
There are several types of clinical research studies, including:
- genetic studies to find the role of genes in different diseases
- prevention studies to test ways to prevent specific diseases
- behavioral studies test how people act in different situations
- physiological studies to increase understanding of how the human body functions
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future.
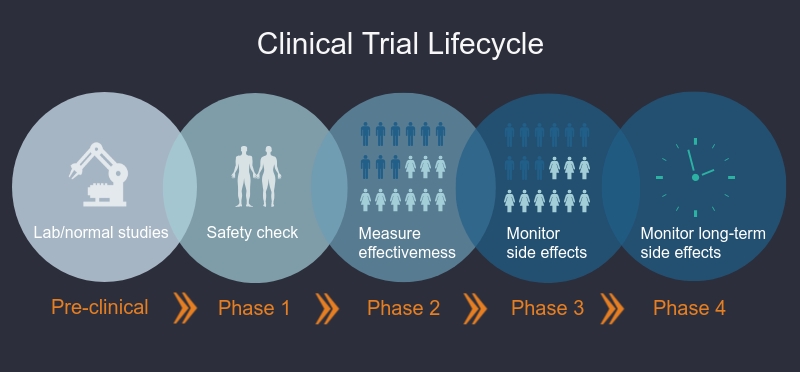
What are the phases of clinical trials?
Clinical trials are done in different phases. In Phase I trials, researchers test an intervention in a small group of healthy volunteers to discover if the intervention is safe, the correct dosage, and if volunteers have any reactions to or side effects from the intervention. In Phase II-Phase IV studies, researchers want to learn more about the safety of the intervention and how well it works in larger groups of participants.
Phase I trials
Researchers test a drug or treatment in a small group of people (20–80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
Phase II trials
The new drug or treatment is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
Phase III trials
The new drug or treatment is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
Phase IV trials
After a drug is approved by the FDA and made available to the public, researchers track its safety in the general population, seeking more information about a drug or treatment’s benefits, and optimal use.