Currect Projects | Overview
Project One: Nuclear receptor mediated ocular neovascularization and inflammation
This project investigates the role of nuclear receptors, specifically a lipid-sensing nuclear receptor RORalpha as a transcriptional regulator of ocular angiogenesis and inflammation. We found RORalpha directly controls transcription of SOCS3, a negative mediator of inflammation, to influence retinal inflammation and retinopathy in a mouse model of oxygen-induced retinopathy.
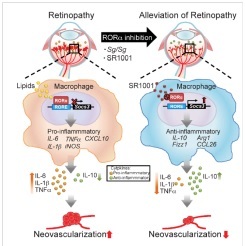
Figure legend: Schematic illustration of a proposed role of RORα in regulating pathologic retinal neovascularization in retinopathy through modulation of SOCS3-dependent inflammation. Schematic drawing of eyes illustrates pathologic neovascularization in retinopathy associated with pro-inflammatory cytokines. Loss of RORα in Staggerer mice (Sg/Sg) increased transcription of Socs3, a negative regulator of inflammation. This led to changes in macrophage function from a pro-inflammatory state to an anti-inflammatory state, with decreased expression levels of inflammatory cytokines (Tnf, Il1b, Il6) and increased levels of anti-inflammatory cytokine Il10, thereby alleviating pathologic neovascularization in retinopathy. Inhibition of RORα with an inverse agonist SR1001 promoted Socs3 expression and protected against retinopathy. Thus targeted inhibition of RORα may represent a new potential therapeutic approach in treating retinopathy.
Project Two: Small non-coding RNAs in retinal angiogenesis
This project investigates the role of small non-coding RNAs, particularly microRNAs in regulating retinal angiogenesis in development and in diseases. One example is miR-150, which we found was specifically enriched in normal retinal vessels and suppressed in pathologic neovascularization. Our findings suggest miR-150 may function as an intrinsic suppressor of ocular neovascularization.
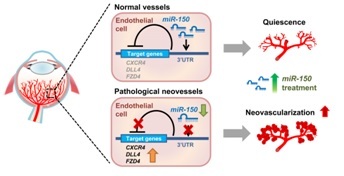
Figure legend: Schematic illustration of an endothelium specific inhibitory role of miR-150 in pathological ocular neovascularization. In normal retinal vessels, endothelial-enriched miR-150 represses the expression of its downstream angiogenic target genes by binding to their 3′ untranslated regions (3′ UTRs) to maintain quiescence of retinal vessels. In pathological neovessels, expression of endothelial miR-150 is decreased, releasing the repression of downstream angiogenic targets (CXCR4, DLL4 and FZD4) and leading to their up-regulation, thereby contributing to formation of pathologic retinal neovascularization. Supplementing miR-150 may compensate for the loss of miR-150 in pathologic neovessels and alleviate pathologic neovascularization in retinopathy.
Project Three: Wnt signaling control of vascular growth and permeability in retinopathy
This project studies canonical Wnt signaling pathway, mutations of which were linked with FEVR and Norrie disease. We are investigating ways to modulating Wnt pathway to prevent or treat these diseases.
